RAL is a world leading scientific research laboratory where researchers study everything from particle physics which are very, very small through to the astronomically large, studying space and the mysteries of the universe.
They host free talks once a month from September to June over a broad range of topics and in this talk, we heard all about plastic eating enzymes.
John McGeehan is a professor of structural biology focused on the global challenge of plastic pollution and his latest work is revealing how an enzyme works to digest plastic allowing it to be recycled.
We were asked the all-important impact question. Which of the following does p e t or pet stand for? Is it plastic eating technology or is it polymer enzyme testing? Is it your pet cat, dog hamster or rabbit? Is it the plastic environment task force or is it something else?
We were given a few seconds to have a guess.
There was a spread of answers, but nobody thought it was their cat, dog or hamster. Which is good because it was unlikely to be that one. The question was answered during the talk.
Professor McGeehan was then invited to tell us all about plastic eating enzymes.
John McGeehan
https://www.port.ac.uk/about-us/structure-and-governance/our-people/our-staff/john-mcgeehan
https://twitter.com/mcgeehanjohn?lang=en
https://www.linkedin.com/in/john-mcgeehan-80230178/?originalSubdomain=uk
https://en.wikipedia.org/wiki/John_McGeehan
John McGeehan is a British research scientist and professor of structural biology. He is director of the Centre for Enzyme Innovation (CEI) at the University of Portsmouth and leads a research team on enzyme engineering.
https://www.youtube.com/watch?v=9QEeoLJQRgU
The following are notes from the on-line lecture. Even though I could stop the video and go back over things there are likely to be mistakes because I haven’t heard things correctly or not understood them. I hope Professor McGeehan and my readers will forgive any mistakes and let me know what I got wrong.
Professor McGeehan thanked us for joining him on a Friday evening (although I’m watching his video on a Monday afternoon).
Professor McGeehan’s research uses facilities at RAL along with his host University, the University of Portsmouth.
He started his talk by explaining a little bit about the background to his work and how we’ve ended up at the current position. The media demonises plastics but Professor McGeehan thinks plastics are really quite an amazing material. They’re just rather misused. Our computer screens and phones at the moment have a huge amount of plastic in them.
The screen, the keyboard and all the parts that that make it work and connect us to the rest of the world. It’s all really amazing technology and we need to remember that plastics are quite essential in order to make these types of things work. This view is slightly controversial.
People often ask Professor McGeehan whether we should go back to glass milk bottles because we know how to wash and recycle them but unfortunately, they’re very heavy and they also break easily. Changing to plastic milk bottles saves a huge amount of energy, particularly the ones shown below, and the standard ones that you get from the supermarket are actually recycled rather well in this country. About 40% of the plastic in those milk bottles is actually recycled material.
We do need to do better at recycling but because the plastic is so light, compared to glass, it saves a huge amount of energy, fuel, transportation and storage of the milk. So actually, on balance, if we can get the recycling part right plastics are actually a really good thing in this case.
We should also think about plastics and their contribution to our health.
The image above shows a little device which is a plastic replacement heart valve keeping a lot of people alive at the moment. These are incredible innovations that come from using materials that are very long lasting they’re durable. They’re very well-known and they’re quite safe.
Of course, that’s not always the case. We see images like the one above on the internet and news all the time where we have huge amounts of plastic used for not possibly the best purpose. Children generate a significant amount of plastic through their toys etc. and parents should be encouraged to buy second hand and minimize plastic waste as much as possible.
It’s actually really tough. These days to reduce the amount of plastic that we use and here’s another controversial one. Plastic packaging
We know if we wrap a cucumber in plastic film it will last for maybe 10 days or two weeks in the fridge and if it’s not wrapped it will simply last a few days so plastic packaging does have a major role to play in reducing food wastage which is a massive problem in this country and around the world. But clearly, we use too much of it and we need to reduce the amount we use and also try and recycle the stuff that we do.
The problems are quite apparent in the image above. It shows a beach in Mumbai a few years ago, and while it can be cleaned up, and it was cleaned up in this case, the problem is not on the beach it’s in the ocean and this is a huge problem as David Attenborough and others have showed us.
https://en.wikipedia.org/wiki/David_Attenborough
Sir David Frederick Attenborough (born 8 May 1926) is an English broadcaster and natural historian. He is best known for writing and presenting, in conjunction with the BBC Natural History Unit, the nine natural history documentary series forming the Life collection that together constitute a comprehensive survey of animal and plant life on Earth.
Professor McGeehan took us back to where plastics originated and you may be surprised to know that plastics were only invented in 1907 the sort of basic synthetic plastics of Bakelite of these pool balls or this phone shown in the image below
https://en.wikipedia.org/wiki/Bakelite
Bakelite (sometimes spelled Baekelite) or polyoxybenzylmethylenglycolanhydride was the first plastic made from synthetic components. It is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. It was developed by the Belgian-American chemist Leo Baekeland in Yonkers, New York, in 1907.
https://en.wikipedia.org/wiki/Leo_Baekeland
Leo Hendrik Baekeland FRSE(Hon) (November 14, 1863 – February 23, 1944) was a Belgian chemist.
Plastics have not been around very long but they’ve had a huge impact on our environment.
Above shows a magazine cover: Life magazine 1955. An American family celebrates the dawn of “Throwaway Living”. Only 65 years ago it celebrates plastic and it’s hard to imagine doing this now.
https://en.wikipedia.org/wiki/Life_(magazine)
Life was an American magazine published weekly from 1883 to 1972, as an intermittent “special” until 1978, and as a monthly from 1978 until 2000. During its golden age from 1936 to 1972, Life was a wide-ranging weekly general interest magazine known for the quality of its photography.
At the time when plastics were invented people thought, oh my goodness we are unchained from the kitchen sink. In fact, the kitchen sink is probably redundant now. Because we can just buy plastic cutlery, plastic plates, plastic cups use it once, throw it away, never need to do any washing up ever again. Technology has saved us from all this drudgery.
Of course, at that point in time, we were not very good at looking forward. We’re good at looking forward a couple of years, or maybe a few governments that last five years or 10 years but looking forward 50 years 100 years we’re classically very bad.
In trying to determine and forecast the consequences 65 years ago we could never have imagined the consequences that plastic pollution would have on our environment.
So, when we think about coming up with new solutions. We need to make sure we’re thinking very, very long term.
Above is a scary graph. The X axis shows years going from 1917 up to 2017. The Y axis is plastic production in millions of tons per year. And what you can see is it’s not even linear it’s going up exponentially. We’re producing about 400 million tons per year of plastic.
The problem is that a lot of this is ending up in landfill and it’s leaking straight out into rivers and then out into the ocean.
It’s estimated there is anywhere between eight and 12 million metric tonnes of plastic waste entering the oceans every year which are really shocking statistics.
The above image illustrates where all the plastic in the world is. The blue bit shows the amount of plastic in the world between 1950 and roughly now. It’s about 8.3 billion tons.
The next part is green and you can see it shows that about three quarters of the plastic was simply used once with the vast majority of it (about 80%) being discarded, just literally thrown in a landfill or thrown away in a dump.
A little bit has been burned to release energy and an even smaller amount has been recycled. Some of it is still in use. For instance, the plastics used in construction, such as that the pipes that take water under the roads, etc..
So that is how plastic in the environment is being used. But what’s really apparent is that over half the plastic is used once and then thrown away, which is incredibly bad.
The other thing we need to think about is how much plastic is worth to people because that really drives how things happen in the world. Unfortunately, plastic is worth a huge amount of money and it’s estimated that the market worldwide is about a trillion dollars.
About 300 million tons of plastic is produced every year. If we carry on at this rate by 2050, which is only 30 years away, about one fifth of the total world’s oil production will be going, not to fuel, but to plastic and overall, we’re only recycling about 20% globally, 25% is burned and about 55% is just thrown away. Nothing recovered at all from that.
And even that 20% recycled is probably an overestimate because some countries consider burning it for energy as recycling, which it really isn’t.
Of course, there are lots of statistics, but the following Professor McGeehan thinks is important and it is about the plastic bottles used for fizzy drinks and water.
About 20,000 are purchased every second, which is staggering, and only about 50% of those are recycled and about 7% of those are turned back into plastic bottles.
Now why is that because we’ve got the technology to do it and the talk explained why that is.
Now the big problem with plastic, of course, is that it ends up in our environment and it lasts a very long time. It was a fantastic but over designed product. Durable and lightweight, but unfortunately very durable.
And this means that that the plastic bottle that’s thrown in the ocean today will still be there in hundreds of years from now, which is really, really scary in terms of the environmental consequences of that.
Above is an image from the National Geographic that should never exist.
https://www.nationalgeographic.com/magazine/
https://en.wikipedia.org/wiki/National_Geographic
National Geographic (formerly the National Geographic Magazine and branded also as NAT GEO) is the official magazine of the National Geographic Society. It has been published continuously since its first issue in 1888, nine months after the Society itself was founded. It primarily contains articles about science, geography, history, and world culture. The magazine is known for its thick square-bound glossy format with a yellow rectangular border and its extensive use of dramatic photographs.
The image shows a plastic cotton bud which should never really have been made from plastic in the first place because as it lasts too long. It could have been made from cardboard or paper, but we could ask does it actually have to exist in the first place. Too many of the things that are sold are really not needed at all. And of course, nature has a very intimate relationship with anything that ends up in the ocean.
It’s very easy to think that plastic waste in the sea is a local problem but it’s not. It’s a worldwide problem.
If we look at the map of the world shown above, we can see orange spots. These show gyres. These are where the ocean currents tend to collect plastic waste that’s dumped from around the world and produce huge plastic islands.
https://en.wikipedia.org/wiki/Ocean_gyre
The five major ocean gyres
In oceanography, a gyre is any large system of circulating ocean currents, particularly those involved with large wind movements. Gyres are caused by the Coriolis effect; planetary vorticity, horizontal friction and vertical friction determine the circulatory patterns from the wind stress curl (torque)
They’re not really plastic islands but more like a soup of plastic. We can see this from a perspective of diver Richard Horner.
https://www.youtube.com/watch?v=31CdhLMV7Es
This was the once pristine island of Bali and you can see they’re absolutely teaming full of plastic.
https://en.wikipedia.org/wiki/Bali
Bali is a province of Indonesia and the westernmost of the Lesser Sunda Islands. East of Java and west of Lombok, the province includes the island of Bali and a few smaller neighbouring islands, notably Nusa Penida, Nusa Lembongan, and Nusa Ceningan.
Richard Horner is a professional diver and photographer and his video shows the typically shocking state of these waters. They used to be crystal clear blue and you can actually see there is all this floating plastic on the top of the surface and this is what we see from the air. But it’s actually much worse below the water. If we start to look at what’s happening under the water. We can see that all this plastic is like a soup.
We see these fragments like the plastic bag shown below but there is a kind of fuzziness in the water.
This fuzziness occurs because there’s lots of tiny micro plastics in the water. These are disposed of down sinks or are formed from larger pieces of plastic breaking down in the water over time.
What is going on here. What is really happening. Why is this PET and little triangle placed on all those plastic bottles? And why has it got a 1 written in the middle of the triangle?
It means it is a number one plastic and the PET means it is a plastic called polyethylene terephthalate
https://en.wikipedia.org/wiki/Polyethylene_terephthalate
Polyethylene terephthalate (sometimes written poly(ethylene terephthalate)), commonly abbreviated PET, PETE, or the obsolete PETP or PET-P, is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, thermoforming for manufacturing, and in combination with glass fibre for engineering resins.
It is used for lots of things, not just plastic bottles, but also fibres for clothing and carpets and it’s one of the most common thermal plastics that we actually use today. That little triangle suggests that it can be recycled. So why are they sitting in these huge landfills.
Well, consider how plastic is made. We’re producing oil and gas and using them to make small molecules called monomers.
https://en.wikipedia.org/wiki/Monomer
Making nylon
A monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
These monomers react and are stuck together with something called an ester bond and this gives us a polyester.
https://en.wikipedia.org/wiki/Ester
In chemistry, an ester is a chemical compound derived from an acid (organic or inorganic) in which at least one –OH (hydroxyl) group is replaced by an –O–alkyl (alkoxy) group.
https://en.wikipedia.org/wiki/Polyester
Polyester is a category of polymers that contain the ester functional group in their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in the cutin of plant cuticles, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. The material is used extensively in clothing.
Scanning Electron Microscope picture of a bend in a high-surface area polyester fibre with a seven-lobed cross section
So, a plastic bottle is made of polyester, as PET is a type of polyester. If you look at some of your clothing, you’ll see that they have a label stating they are made of polyester too.
So, all plastic originally comes from oil and gas production. The reason why it ends up in landfill is actually because of something called down cycling and once it ends up in landfill, if it is not very well managed it will end up polluting the environment.
This is because we’re operating in something called a linear economy.
Basically, we start by digging something up, producing something out of it, shipping it around the world, using it once and then throwing it away. So, it’s not very ethical or environmentally friendly and even if we can recycle this plastic, we often just melt it down to make lower value things like fibres for t-shirts and other cheap clothes. The plastic starts to lose some value over time.
Eventually, the plastic might end up as carpet, which is really low value (however I like my mancave carpet) because no one wants to recycle it because there’s no money in it. So even though it’s sort of being recycled. It’s inevitably going to end up in landfill eventually.
What’s not happening here. It’s not being recycled into plastic bottles. It’s been downcycled, and it’s just a matter of time before it ends up being in the environment. So that’s something we really need to change.
Taking a closer look at this.
https://www.ted.com/talks/emma_bryce_what_really_happens_to_the_plastic_you_throw_away?language=en
We start digging up oil and gas.
https://www.world-petroleum.org/edu/222-how-do-we-get-oil-and-gas-out-of-the-ground
You can see oil and gas is the start of the process.
Oil and gas can move through the porous rocks (rocks with gaps between the grains). The oil and gas move upwards from the source rock where they were formed. When they met a layer of cap rock (a rock with no spaces between the grains) the oil and gas are trapped.
Oil and gas get trapped in pockets underground such as where the rocks are folded into an umbrella shape.
A well is drilled so that the crude oil and other liquids travel up the bore hole. When it comes to the surface the crude oil has to be moved closer to where it is needed.
Finding oil and gas trapped deep underground and drilling a well are very complicated and expensive. It costs millions of pounds to drill a well and only a few are successful. The liquids found underground can be a complicated mixture of water, crude oil and gas. The crude oil and gas need to be separated before they can be transported safely.
Oil and gas are often found far away or under the sea. They have to be transported to an oil refinery. This is often through a pipeline or in a tanker.
The crude oil is often found in remote places such as deserts, jungle or the Arctic. Transport of the crude oil to the refinery is sometimes very complicated. The oil refinery turns crude oil into useful products and materials. These are transported all over Britain or abroad. The products can travel through pipelines, by road, rail or by boats around the coast or along rivers and canals.
https://www.world-petroleum.org/edu/223-how-is-crude-oil-turned-into-finished-products-
https://www.youtube.com/watch?v=p5fjb1oA7No&vl=en
The oil and gas are sent to refinery and chemical works in order to produce the molecules called monomers.
And then, as mentioned before, these monomers join together to form long chains. Which are then turned into polyethylene terephthalate pellets.
Occasionally the pellets fall off ships and end up on beaches. But normally, they’re melted down and blow moulded into plastic bottles.
https://www.youtube.com/watch?v=weR31x9HZDs
So, these are the single use plastic bottles that we have been spoken about. They will be filled with their fizzy drinks or water.
Once the liquids have been used the bottles often just get tossed into the bin. This is a really terrible way of dealing with things and we need to really get better at this.
Let’s go back to the landfill.
So that’s the life cycle of a plastic bottle and we really want to improve things because we’ve done a really unfortunate experiment because we’ve effectively taken a huge amount of plastic and put it in the environment for 50 years plus.
Now nature is incredibly powerful and it is evolving mechanisms to deal with the plastic, but much slower than we are producing plastic.
In 2018 a PET recycling facility in Japan found bacteria that had actually evolved to live off the plastic bottles and this is really stunning. This is nature in action.
This false-colour SEM (scanning electron microscopy) image shows Ideonella sakaiensis. Image credit: Shosuke Yoshida et al.
Above is a picture of the bacteria actually living off the surface of the plastic and what they’re doing is creating a couple of enzymes. The enzymes are breaking down the plastic into monomers again and the bacteria are eating the monomers as a food source to give them energy. This is environmental and bacterial selection.
This is really amazing. You’ve got a landfill site with all these plastic bottles and lots of bacteria that usually live off the little bits of sugar that get left in the bottles from sugary fizzy drinks. Then suddenly a mutation happens within one of those bacteria and suddenly it has an appetite for plastic. It’s got this enormous food source that no one else can use.
If we look hard enough in the environment will probably find this happening all over the world in types of plastic, which is really exciting.
An enzyme is like a little mini factory that speeds up reactions and, in this case, it is breaking things down. You can also get enzymes that make things.
https://en.wikipedia.org/wiki/Enzyme
Enzymes are proteins that act as biological catalysts (biocatalysts). Catalysts accelerate chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyse individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual ‘pseudocatalytic’ properties.
We use enzymes, all the time. In the image below a man drops pasta sauce down his shirt, which is a biological stain, and uses a biological washing powder containing enzymes to wash it clean.
These enzymes originally came from the environment, from fungi and bacteria, and we’ve been able to reproduce them and make use of them every day because enzymes are really all around us. They’re not anything very new and there are many different things that they can be used for, including cosmetics and biofuels.
Fats and oils can be broken down with enzymes into sugars which are then turned into bioethanol to provide “petrol or diesel” for cars. Enzymes are also used in the pharmaceutical industry to produce drugs.
Enzymes are used all over the place. And we’re very good at making them and that’s really important. Their usefulness will be explained shortly.
What’s the bacteria in the rubbish dump doing?
The bacteria are sticking to the surface of those plastic bottles so creating enzymes and digesting the plastic, and they’re producing basically their favourite food. PET. These bacteria have literally evolved to eat plastic and this was a really big discovery and this is what Professor McGeehan is investigating. The bacteria are taking these very long chains PET molecules and breaking them down into small chemicals which are exactly the same ones that we currently get from oil and gas to make plastic.
Where did PETase come from, how does it work and can we make it faster?
So, you can see if we can use these enzymes to generate these monomer molecules then we don’t need oil and gas to make them anymore. We can use waste plastic to make plastic or even better materials.
The idea is that we take the waste plastic with these long chains of plastic polymers and we pour in the enzyme, just like adding biological washing powder to a washing machine. The long molecules are broken down into the monomers, which are purified, polymerised and turned into new plastic products.
The great thing is that, unlike melting plastic, the material won’t go grey and gloopy where you can’t really use it for some products. You don’t lose some of its nice properties. Using enzymes gets all the nice properties back again. So, it’s like brand new plastic and you could literally repeat this process for infinity and never need to go back to the oil and gas again, which is incredibly exciting.
To try and work out what is happening we need to see what is happening on the surface of a plastic bottle.
Professor McGeehan is working with some researchers in America at the National Renewable Energy laboratory.
https://en.wikipedia.org/wiki/National_Renewable_Energy_Laboratory
The National Renewable Energy Laboratory (NREL), located in Golden, Colorado, specializes in renewable energy and energy efficiency research and development. NREL is a government-owned, contractor-operated facility, and is funded through the United States Department of Energy. This arrangement allows a private entity to operate the lab on behalf of the federal government. NREL receives funding from Congress to be applied toward research and development projects. NREL also performs research on photovoltaics (PV) under the National Center for Photovoltaics. NREL has a number of PV research capabilities including research and development, testing, and deployment. NREL’s campus houses several facilities dedicated to PV research.
NREL’s areas of research and development are renewable electricity, energy productivity, energy storage, systems integration, and sustainable transportation.
Using a really powerful electron microscope we can zoom in about 3000 times magnification onto the surface of the plastic bottles.
The enzyme is starting to munch away at the plastic and it’s creating these holes. If left long enough the plastic would disappear and what we’d be left with would be a clear solution of monomers and these monomers could then be used again and again to make more plastic.
This is really quite exciting from the point of view of recycling plastics. It’s a very efficient process and works at about room temperature as well.
No weird experimental conditions are needed. It can just take place on a laboratory bench although the process does need to be scaled up.
So how does Professor McGeehan intend scaling the process up?
He and his colleagues published their first study on the enzyme in 2018 and the media went a bit mad with the news. It was a bit of a shock for all the researchers involved especially when they found themselves on the news.
What was particularly pleasing was that the announcement came out about the same time as David Attenborough’s blue planet programme came out.
https://en.wikipedia.org/wiki/The_Blue_Planet
David Attenborough has really helped us to understand what a massive impact these plastics are having on the environment. So, in some ways, it wasn’t surprising that people were looking for solutions.
The large media coverage actually helped to get the message across to the world that enzymes have real potential to do this.
https://www.pnas.org/content/115/19/E4350?source=post_page—–fe4e88e7e4c2———————-
This is the paper that was published by Professor McGeehan’s PhD student Harry Austin, who became quite famous because of it. It’s been downloaded more than 90,000 times (150,000 times in fact). It’s one of the top papers of 2018 and has informed government strategy.
Very big teams were involved with the research. 21 people were named on the paper. And since the paper was published the teams have got a lot bigger. It takes an awful lot of people to do all the experiments that are required for this type of experiment.
A few of the 21 people mentioned on the paper.
What goes on in the lab
https://www.youtube.com/watch?v=Y-GqbPmSuj4
The video shows some of the facilities used in the research and the processes involved.
The enzyme is removed from the bacteria and taken to the lab. A SEM is used to monitor the process.
https://en.wikipedia.org/wiki/Scanning_electron_microscope
A scanning electron microscope (SEM) is a type of electron microscope that produces images of a sample by scanning the surface with a focused beam of electrons.
The gene in the bacteria responsible for making the enzyme is transplanted into a lab strain of bacteria that allows lots of the enzyme to be produced. The enzymes are turned into crystals.
https://phys.org/news/2018-05-enzyme-degrades-plastic.html
PETase crystal is shown in blue, with PET chain (yellow) bound to active site, where it will be degraded. Credit: Rodrigo Leandro Silveira
https://www.youtube.com/watch?v=UqjWyQjyB9o
https://www.youtube.com/watch?v=gALzc1mQ3C4
Professor McGeehan uses X-rays in his lab and the synchrotron at RAL to investigate these crystals and generate the 3-dimensional model of the PETase enzyme
https://en.wikipedia.org/wiki/X-ray_crystallography
X-ray crystallography (XRC) is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information.
https://en.wikipedia.org/wiki/Synchrotron
A synchrotron is a particular type of cyclic particle accelerator, descended from the cyclotron, in which the accelerating particle beam travels around a fixed closed-loop path. The magnetic field which bends the particle beam into its closed path increases with time during the accelerating process, being synchronized to the increasing kinetic energy of the particles.
https://en.wikipedia.org/wiki/Synchrotron_radiation
Synchrotron radiation is the electromagnetic radiation emitted when charged particles are accelerated radially, e.g., when they are subject to an acceleration perpendicular to their velocity. It is produced, for example, in synchrotrons using bending magnets, undulators and/or wigglers. If the particle is non-relativistic, the emission is called cyclotron emission. If the particles are relativistic, sometimes referred to as ultrarelativistic, the emission is called synchrotron emission. Synchrotron radiation may be achieved artificially in synchrotrons or storage rings, or naturally by fast electrons moving through magnetic fields. The radiation produced in this way has a characteristic polarization and the frequencies generated can range over the entire electromagnetic spectrum, which is also called continuum radiation.
https://en.wikipedia.org/wiki/Synchrotron_light_source
X-ray diffraction (XRD) and scattering experiments are performed at synchrotrons for the structural analysis of crystalline and amorphous materials. These measurements may be performed on powders, single crystals, or thin films. The high resolution and intensity of the synchrotron beam enables the measurement of scattering from dilute phases or the analysis of residual stress. Materials can be studied at high pressure using diamond anvil cells to simulate extreme geologic environments or to create exotic forms of matter.
https://en.wikipedia.org/wiki/Diamond_Light_Source
Diamond Light Source (or Diamond) is the UK’s national synchrotron light source science facility located at the Harwell Science and Innovation Campus in Oxfordshire. Its purpose is to produce intense beams of light whose special characteristics are useful in many areas of scientific research. In particular it can be used to investigate the structure and properties of a wide range of materials from proteins (to provide information for designing new and better drugs), and engineering components (such as a fan blade from an aero-engine) to conservation of archaeological artifacts (for example Henry VIII’s flagship the Mary Rose)
So PETase is the enzyme that digests PET and is the one that the bacteria produced in that rubbish dump.
https://en.wikipedia.org/wiki/PETase
All of the experiments have shown the molecular workings of the enzyme
You can see the structure and individual atoms and when you get to that level of detail you can actually start to understand how the enzyme works.
One of the first things they did was to compare the enzymes.
They looked at the top left PETase and were really surprised to find that looked exactly the same as a Cutinase enzyme, which was really quite surprising.
Cutinase enzyme is a bacterial enzyme that’s used for burrowing through plant leaves and this didn’t make sense to them at first. But when looking into this a bit more detail it became clearer.
This is a surface of a kale plant leaf.
If you take any plant leaf and run water over it you will see that the surface has a kind of waxy coating that allows the water to run off. This is because nature has generated a natural polyester called Cutin, and most green leaves surfaces are covered in it. And
It’s basically nature’s polyester. It’s nature’s plastic and bacteria have evolved over millions of years to eat through it to get into the nice juicy plant material in order to live off it.
It’s all to do with evolution and what’s happened in this case is something really spectacular.
Below is a close up of the Cutinase enzyme. The active-site jaws of a leaf polyester-digesting enzyme.
The cavity, cave-like structure, at the top is where the chemistry happens. This is the jaws of the enzyme which can break things apart. This is where all the action happens
This enzyme developed over billions of years to eat through the natural polyester Cutin.
Now a few mutations, just a few small changes to the DNA, changes one of the amino acids and opens up the active site, widens the jaws, to give the enzyme the ability to work on synthetic polyester. PET.
We can start to understand how this enzyme has evolved to eat plastics. Basically, if we leave the plastic in the environment long enough, we will have these random mutations happening and just occasionally one like this happens that allows it to have a new facility and new activity.
We are now able to see the real molecular details.
This yellow chain here is PET and we can actually see it embedded in the enzyme and figure out how it works. This is really exciting because it allows the researchers to engineer and even faster enzyme.
So, what they decided to do was to turn the enzyme back into a plant eating enzyme by changing its amino acids.
They turned it into what they thought would be a really rubbish enzyme for PET and a really great enzyme for eating plants.
The results showed how little they understood the enzymes because when they did the experiment, rather than the enzyme stopping eating the plastic it actually ate plastic faster.
They figured out why, eventually.
The above left image shows the plastic before any enzymes were used. The above centre image shows the results of the natural enzyme from the rubbish dump on the plastic surface and the above right image shows the plastic surface after the engineered enzyme was used, which was about 20% faster.
This is a great result because it tells us that the enzymes that nature has produced aren’t fully optimized yet so we can actually make them faster still, and this is what they’re trying to do in the lab.
How much faster can they make them. Well, actually, that bacteria in the plastic rubbish dump actually makes two enzymes.
And they’ve recently solved the second enzyme structure and the data should be published next week or the week after.
This enzyme, if you add it to the PETase makes the process about twice as fast, so not 20% but 200% and that’s really, really exciting. They’re obviously very happy about this.
The team then started thinking whether there was anything more they could be doing.
What they were able was to take the PETase enzyme (light blue colour in the image below) and the new enzyme (red colour in the image below) and basically link them together to make a super enzyme
Image credit: Aeron McGeehan
Professor McGeehan didn’t want to give the game away before the publication in a week or two, but he did say that it works and it works faster. And that’s really, really exciting because this means they can start engineering much faster enzymes.
The image above shows some of the Professor’s PhD students (Tom, Harry, Rosie and Dan) and they’re really working hard on a whole number of enzymes to try and design them to be faster and better
The enzymes can be made faster. Help towards this aim can be found in interesting places. The image below was taken in Yellowstone National Park.
https://en.wikipedia.org/wiki/Yellowstone_National_Park
Yellowstone National Park is an American national park located in the western United States, largely in the northwest corner of Wyoming and extending into Montana and Idaho.
The images show some of the hot spring geysers, and we can see some amazing colours in them. The reason for these colours is because of the bacteria that live there and they’re living at very high temperatures. We know that at higher temperatures reactions happen more quickly.
So, they collected about 100 enzymes from here and other places and they’re currently looking at them in the lab, testing to see if they eat plastic faster and whether they will work on other types of plastic besides PET.
So, coming back to the original problem. It’s the horrible linear economy
Can they improve this situation with enzymes? Yes, they can.
Basically, instead of going linearly, we go around in a circle.
We make single use plastic bottles and then we use enzymes to turn them back into monomers.
We go round and round infinitely in this circular economy model which is incredibly sustainable compared to using fossil fuels.
This does a number of things. It reduces the amount of landfill and downcycling as well as reducing the amount of environmental pollution.
It stops us needing oil and gas to make more plastic. We’ve got enough of it up here on the surface already.
Needing less oil and gas will reduce greenhouse gases which, as you know, are really a major contribution to climate change. Remember that oil and gas are non-renewable too.
We decouple the cost of plastic from the cost of oil.
We stop downcycling in landfill.
We can recycle infinitely
We can upcycle
We can turn waste into a valuable commodity
We can even take those monomers and add new monomers to them and make even better plastic items. Things like carbon fibre for super light cars or wind turbine blades.
There’s a lot of potential here and we need a lot of different types of scientists to work on the research.
https://www.port.ac.uk/research/research-centres-and-groups/centre-for-enzyme-innovation
The government liked what they were doing and gave them 6 million pounds to hire 15 new researchers from across the world. Professor McGeehan was very happy that he was able to bring all these people together in brand new labs, which unfortunately had to close for a bit due to Covid-19, but they are all working hard now on the classic enzymes.
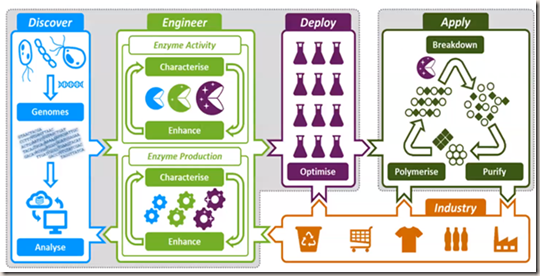
The above image summarises what they are doing. They’re going out into nature and trying to discover (the bit in blue).
They’re trying to discover bacteria from weird and wonderful places and take them into the lab. They will grow them, remove their DNA and find the enzymes and then engineer them to be fast. They’re using things like the diamond light source and all those x ray experiments to investigate their structure.
Then they make lots of them and start working with industry to find out what they need, what types of plastics they’re using, which ones they need in the future and try and make them more sustainable.
One of the issues people have raised is that just making these enzymes in the lab is not enough to tackle a global crisis and they are absolutely right.
They need to think about making these enzymes on a huge scale. Something the pharmaceutical industry does already.
Glaxo Smith Klein offered a laboratory just down the road from the university where they already make penicillin in huge bioreactors.
This bottle of PETase enzyme was made at GSK
As part of their contribution to the environment they’re helping with this project. It’s really exciting that this is happening because not many areas of science get such support from companies.
Of course, there isn’t just PET plastic but all the other plastics out there. PET is number one, but there are lots of others.
Polyethylene plastic bags and those films on those microwave trays. PVC is a really difficult one to break down and is actually pretty toxic as well. So, we need to be quite careful how we do that.
Polypropylene plastic straws end up underground. These are really hard plastics to break down. Polystyrene is another big polluter
And of course, at the moment there are disposable masks. They’re actually made of woven polypropylene. So they could end up in the ocean. They will be there for hundreds of years.
Try and use cloth ones that you can rewash because the disposal ones are going to give us a huge problem.
Researchers need to be looking at enzymes for other types of plastics and they are starting to find them working with other teams of researchers around the world. They can really make some progress here.
The University of Portsmouth has a new project called revolution plastics, which is bringing together scientists, engineers, economists, environmentalists and even psychologists who understand the behaviour of people in order to tackle the plastic problem.
https://www.port.ac.uk/research/themes/sustainability-and-the-environment/revolution-plastics
As a lob the Portsmouth team need to work together with researchers across the world. A very exciting prospect. Also, they need to work with discovering how different types of places work with industry really closely because they don’t want to design a solution that’s worse than the problem. They have to work with the government and really think about what they’re doing.
There are lots of things we can do to reduce the amount of plastic we use. Do we actually need the plastic item? We can reuse an awful lot of it and actually we can recycle much more than we actually do. We can lobby our local councils to have much more of our plastic recycled. So, every single one of us can make a real difference. And I think if we all come together, we can really make a massive impact.
Professor McGeehan said he had so many people to thank as he did not do very much of the science. He actually manages a big team of about 3030 scientists currently at the University of Portsmouth, which includes the team working on the enzyme
There are also teams of researchers in other parts of this country and around the world including the diamond light source here in the UK and the University of South Florida.
Professor McGeehan also thanked the people who produced the amazing images because they helped to get the information across.
He also thanked the people who provided the funds for the research,
Questions and answers
1) Could the bacteria escape and attack plastics, which are still in use?
That’s a great question. And it’s one that gets asked quite a lot. The thing is, this is happening already in the environment. The original enzymes that were discovered were from bacteria that had evolved naturally in a rubbish dump. We don’t need to worry about it too much at the moment because these enzymes are very, very slow and the bacteria grow very slowly. And we can see that because plastics last so long in the environment we don’t need to really worry too much about bacteria digesting things that are really important to us. Also, the enzymes that we’re using at the moment and the laboratories that we plan to use along with recycling facilities are non-living. They don’t reproduce and they biodegrade quite happily and safely. So, in that respect. It’s quite a safe technology.
2) How do you protect plastic that you want to keep for a long time, e.g. coverings of wires and cables and electronics.
Well, most of those plastics that are used for these sorts of applications are incredibly difficult to break down. So, it’s very unlikely that bacterial will be able to eat them anytime soon. The only way you can really break these down is to bring them into a recycling facility near where you live and add really huge, concentrated amounts of enzyme in a very small volume. That’s not really going to happen at the moment so we don’t need to worry about protecting those plastics. In fact, we have a problem because they’re so durable. They won’t break down naturally
3) Are the bacteria harmful at all in any way to living things?
We don’t think so. There’s lots of studies going on at the moment including investigations into the harm caused by micro plastics to humans and animals. We know microplastics get into our bodies but we don’t know of any harm, yet.
Some of the plastics release chemicals when they break down in the environment and some of them aren’t very nice at all. The plasticized flame retardants and dyes are horrible things. So, it’s actually really important that we get these plastics into a recycling facility in the first place. But in terms of the bacteria we know, they’re natural and the world is covered in them, particularly in places like recycling centres. So, there’s nothing biological to worry about.
4) Would the enzymes work on clothes and carpet.
Yes, they do. And in fact, there is a company in France that’s doing really fantastic work with some thermotolerant enzymes. Enzymes that work at high temperatures.
You can take fleeces, for example, which have lots of PET in them and you can add enzymes to them to break them down into monomers and then turn them back into any sort of plastic you want.
They’re still in the early stages of doing this on a commercial basis, but commercial plants are starting to be built now, which is really exciting. So yes, we could tackle textiles, which is a huge waste and ends up in landfill and it would be great to be able to do that.
5) I think you’ve partially answered the next question. When do you think the enzymes could go into use and how long until this has a real impact on the environment?
When Professor McGeehan was first asked that question in 2018 the answer was at least five or 10 years before we see any commercial plan being started because you need so much investment and technology. But it’s being built now and they’re already at one tonne a day digestion processes. Hopefully these processes prove to be worthwhile and economically viable.
Often you get lots of benefits from scaling things up in terms of the economics and it’s looking pretty good at the moment. One of the things that’s really interesting is that it’s not just about making money out of plastics, but looking at the energy savings. Making plastics with recycled enzyme technologies you save about 70% of the energy from making plastic from petroleum and gas. So there’s lots of reasons to take up this technology.
6) You showed the exponential growth of plastic production until 2017. Has there been a significant reduction in the amount of plastic being produced in the years since then?
I would love to say that was the case but I’m afraid I’ve got some very bad news. Not only has it increased, but the rate has increased as well. So not only are we making more of it. But we’re making more of it faster.
And the appetite for plastic as the world needs material goods is exploding at the moment. So, we really need to think very carefully about how we try and mitigate the waste plastic that will result from this
7) How do you fully separate the enzymes from the monomers to make the product safe.
That’s really interesting. So, the reaction basically involves taking a bucket and putting the PET in it Then you pour in some water and some powdered enzymes and then you mix it over a number of hours. Eventually you get this clear solution of monomers with some insoluble monomers. Add some acid or base to change the pH and some of those monomers will literally fall out of solution. You can collect them at the bottom, filter them out and then purify them. Now, that’s what you can do in the lab on an industrial basis. Portsmouth are very, very good at this. Remember that we’re already purifying those monomers from much trickier materials. So, if you look to the big companies like BP you will see that they’ve got all the technology in place to do all of that really quite easily. So not only can we make enzymes easily because the technology exists, but we can also purify all the bits and pieces quite easily as well. So that’s good news. We’re not inventing new technology to do all of this.
8) Are there any enzymes that break down polyethylene?
It has been reported that some bacteria have been seen to do this. You may have seen some stories about wax worms, moths and larvae doing this. So, enzyme eating plastic bags, the bags being one of the major sources of polyethylene pollution, are currently being hunted to see if we can make them in the lab, but clearly, it’s happening in nature.
Unfortunately, it’s happening quite slowly but the researchers have got a few.
9) It’s brilliant. It’s nice to know there’s always something in nature that you can copy and you’ve talked about speeding up the action of the enzymes. How much faster, would it have to be in order to compete economically with the use of oil.
Well it depends how much oil is taxed and how much is left under the ground/sea. We might have to start drilling deeper, which will increase its cost and whether we want to ruin a pristine environment. We need to think whether this is really the right way to go. So, what they’re doing in the lab is to make the enzymes very cheap and you can make enzymes very cheaply indeed.
The technology exists and they’re building a plant in Leon in France to do this economically. It is looking very promising and they are hoping to be able recycle PET. The PET that comes out of those plants will be a little bit more expensive but not hugely expensive and if lots of the big companies, that use the plastic, become involved the price will drop and then it will become as reliable as oil and gas made plastics.
The problem, of course, is that oil is very cheap at the moment. So, these enzyme technologies need to be even cheaper still but I think we can do it.
10) How long does it take to break down a plastic water bottle, for example, at the moment, with the technology that you’re working on?
The enzyme that was developed in France recently was able to take the cleaner rubbish type plastic bottles directly from a recycling plant, put them in the reactor and digest them almost completely in about 10 hours.
As we start to develop even better enzymes, I think we’ll get the process down to a few hours and then it really starts to become commercially viable at that point. It’s already commercially viable at 10 hours, I would say.
11) Can the bacteria degrade the plastic whilst it’s in use e.g. a plastic bottle of water. If the bacteria degraded the plastic wouldn’t it cause plastic contamination of drinking water.
Actually, industry is very good at keeping things sterile using high temperatures and UV light to kill bacteria.
There’s always a risk of bacteria getting into drinking water, but there’s no more reason for these bacteria to be a problem as they actually grow rather slowly on plastic. They much prefer the sugar on the surface, to be honest. So, they only start using the plastic if the sugar runs out.
They are quite slow growing in the environment. It’s not something that would necessarily cause any biological harm or disease or anything like that. So, from that point of view, we shouldn’t be too worried
12) If you were to salvage plastic from the oceans, would the enzymes be affected by the salt water.
Interestingly some of the conditions that we find these bacteria in are quite salty anyway, so, we can breed the enzymes to have different properties. For example, we can make them resistant to heat, acid and salt. They’ve done those sorts of experiments in the lab already with other types of enzymes quite successfully as have others research groups.
This is one of the reasons why we want to work with industry so closely because we want to find out what’s the easiest thing to do. Should we clean the plastic first or should we make enzymes that can deal with really dirty plastic because the problem is that most of the plastic waste is mixed up and contaminated. So being able to use enzymes that can pick out just one type of plastic from the mixture is actually quite a possibility to reduce the amount of sorting that we need to do.
So, we’re looking into mixed plastic waste to see if that’s something that these enzymes could deal with.
13) Could the enzymes be used to create energy as a by-product to breaking down the plastics?
Interesting. There are people working on this. Our lab is not doing those experiments, but it’s a great idea. You can produce biomass, carbon dioxide and sugars which can be used for energy. So, another route is to use the bacteria to convert the plastic directly into either energy or some other very useful chemical besides PET. There are research groups around the world looking at doing that.
14) We’ve got two more questions left so the end is in sight.
So, would the enzymes be used on site at garbage islands and landfill or would it need to be collected beforehand and treated separately?
00:58:35Yeah, that’s, that’s a good question. And people have asked could you just add the enzyme to the ocean
Unfortunately, not as that would make the enzymes too dilute. We really need to collect the plastic first. We need to bring all the plastic together in a recycling facility under controlled conditions to make this enzyme really work at its full potential. So unfortunately, this enzyme technology does not solve the problem of collecting the waste in the first place.
We need to get that plastic back out of the ocean and to energy recycling plants but probably more importantly, at the moment, we need to be able to capture it before ends up there in the first place.
15) Cool. And our last question is, are any of the oil companies engaging with you about this?
Now this is a really interesting question because I would say that in some cases the oil companies are actually more interested in this than the recycling companies. The recycling companies are working on very thin margins and they have plants that they’ve invested in for many decades which makes them a little resistant to change.
The oil companies recognize that their main product is not sustainable and they’ve got all the technology and know-how to actually develop technologies for recycling. So, most of the big oil companies have actually engaged with us and we’re in talks with them at the moment to see how we could work together.
I think that it’s a really good green news story that these companies are really engaged.
16) How did you find the bacteria?
We didn’t. It was discovered in a Japanese recycling centre and basically. You can imagine this large pile plastic bottles.
They just scraped around in the soil and the waste water runoff and took the samples back into the lab, based in Kyoto, and tried to see if anything from the samples grew on pure plastic.
It’s not so difficult. We can now go out into the field and start scraping our own samples In fact, some of our students are doing that in all sorts of weird and wonderful places, trying to find bacteria from the natural environment.
17) Thank you, John that’s all of the questions answered for now. That was absolutely fantastic, thank you everybody for all your questions. I think we’ve covered everything